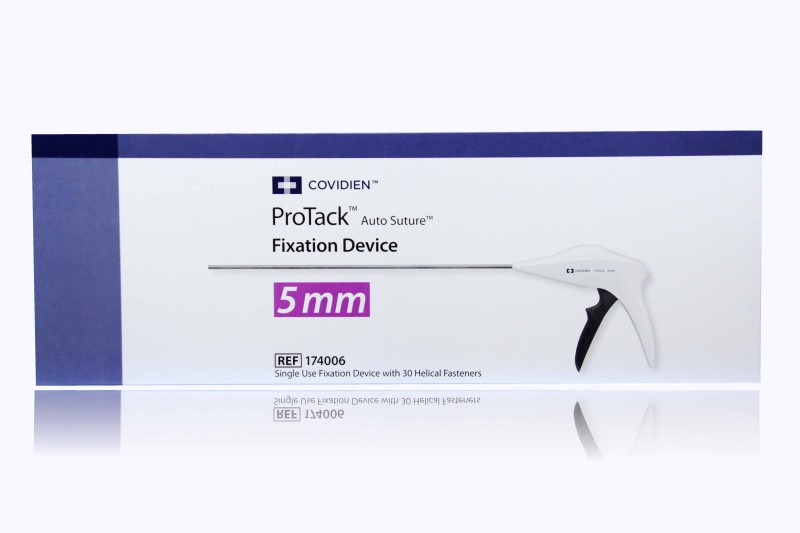
Medtronic Protack 5mm with 30 Titanium Helical Fasteners
In Stock: 218 Eaches
Express: Need it tomorrow? Order in next 2 hours 8 minutes and choose overnight shipping at checkout.
Description
Medtronic ProTack 174006 Hernia Mesh Fixation Device
- Item #: 174006
- Manufacturer: Medtronic
- Medtronic Protack 5mm with 30 Titanium Helical Fasteners
The ProTack™ 5mm Fixation Device is a sterile, single use device for fixation of prosthetic material, such as hernia mesh, to soft tissue. The tack is helical and made of titanium. The overall length of the shaft is approximately 35.5cm. ProTack™ comes in a 30 tack configuration.
The ProTack™ is a single use instrument that fires 30, 5mm titanium helical fasteners.
Additional Details
- Company Name: Covidien LP
- FDA Product Code: GCJ
- FDA Product Code Name: Laparoscope, General and Plastic Surgery
- GMDN Term Code: 45612
- GMDN Term Name: Soft-tissue/mesh anchor, non-bioabsorbable
- GMDN Term Definition: A non-bioabsorbable device designed to be implanted into ligaments or other soft tissues to serve as a site of attachment for surgical binding materials/implants (e.g., mesh, sutures); it is not intended to anchor soft tissues to bone, nor repair cartilage, and is not intended for ophthalmic use. It is typically a threaded screw-like device, and is made of a material that cannot be chemically degraded or absorbed via natural body processes (e.g., metal, polypropylene); it is intended to create a fixed anchor point and is not a suture-based fastening device such as a T-fastener. It is typically implanted with the use of a specialized disposable applicator which may be included.
Additional information
| Size | 5.00mm |
|---|---|
| Arms | N/A |
| Manufacturer | |
| Type | Tackers |
| Unit | box of 1 |
- FDA Product Code: GCJ
- FDA Product Code Name: Laparoscope, general & plastic surgery
- GMDN Term Code: 45612
- GMDN Term Name: Soft-tissue/mesh anchor, non-bioabsorbable
- GMDN Term Description: A non-bioabsorbable device designed to be implanted into ligaments or other soft tissues to serve as a site of attachment for surgical binding materials/implants (e.g., mesh, sutures); it is not intended to anchor soft tissues to bone, nor repair cartilage, and is not intended for ophthalmic use. It is typically a threaded screw-like device, and is made of a material that cannot be chemically degraded or absorbed via natural body processes (e.g., metal, polypropylene); it is intended to create a fixed anchor point and is not a suture-based fastening device such as a T-fastener. It is typically implanted with the use of a specialized disposable applicator which may be included.
Covidien AutoSuture
Covidien’s AutoSuture business now resides within Medtronic’s Surgical Operating Unit, under the Medical Surgical Portfolio. This group is focused on delivering advanced technologies for minimally invasive surgery, with a deep legacy in surgical stapling, energy devices, and laparoscopic instrumentation.
Key products from the former AutoSuture line include:
- Endo GIA™ staplers and reloads – a flagship line of linear cutting staplers used in a variety of laparoscopic and open procedures.
- Signia™ – a powered stapling platform offering real-time feedback and adaptive firing technology.
- LigaSure™ – a trusted vessel sealing system for tissue fusion and hemostasis.
- VersaOne™ trocars – for secure and versatile access during minimally invasive surgery.
- Sonicision™ – cordless ultrasonic dissection tools that combine energy and precision.
These technologies form the backbone of Medtronic’s minimally invasive surgical portfolio, originally developed under the AutoSuture brand by U.S. Surgical Corporation, then acquired by Tyco Healthcare, which later became Covidienbefore merging with Medtronic in 2015. Today, Medtronic continues to build on this heritage, delivering solutions that enhance efficiency, precision, and outcomes in the operating room.