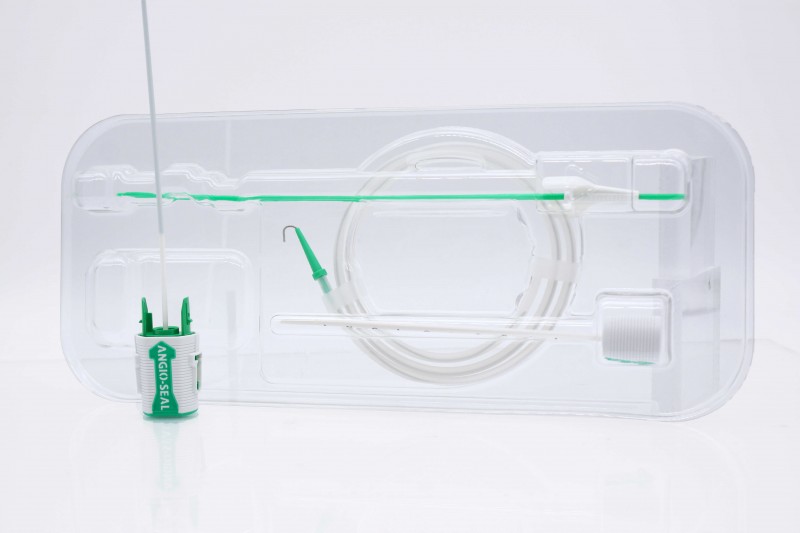
Terumo Angio-Seal VIP Platform Vascular Closure Device 6F
In Stock: 395 Eaches
Express: Need it tomorrow? Order in next 2 hours 8 minutes and choose overnight shipping at checkout.
The item listed above is expired and may be used for educational, training, and non-clinical research purposes only. Any product information appearing below, including the product indication statement, pertains to an in-date item only.
Description
Terumo Angio-Seal™ VIP VCD Vascular Closure Device
The Angio-Seal™ VIP vascular closure device quickly seals femoral artery punctures following catheterization procedures, allowing for early ambulation and hospital discharge. The device creates a mechanical seal by sealing the arteriotomy between a bio-absorbable anchor and collagen sponge, which dissolve within 60 to 90 days. The VIP (V-Twist Integrated Platform) is a unique technology providing a larger collagen footprint for better arteriotomy coverage and designed for enhanced conformability around the artery for a more uniform and secure seal.
Additional Details
- Company Name: Terumo Medical Corporation
- FDA Product Code: MGB
- FDA Product Code Name: Device, Hemostasis, Vascular
- GMDN Term Code: 60710
- GMDN Term Name: Femoral artery closure plug/patch, collagen
- GMDN Term Definition: An implantable, bioabsorbable device designed for haemostasis/closure of a puncture site, through pressure/compression, on a patient having undergone femoral artery catheterization; it is intended as an alternative to manual compression or surgical techniques to reduce the time to haemostasis. It consists of an animal-derived collagen plug that is implanted using an included delivery device (e.g., guidewire, hand-held delivery unit) onto the extravascular surface of the femoral artery access site (top of arteriotomy site), and may be held in place with an included synthetic, bioabsorbable patch/anchor/suture to achieve haemostasis.
Additional information
| Size | 6.0F |
|---|---|
| Arms | N/A |
| Manufacturer | |
| Type | Vascular Closure |
| Unit | eaches |
- FDA Product Code: MGB
- FDA Product Code Name: Device, hemostasis, vascular
- GMDN Term Code: 60710
- GMDN Term Name: Femoral artery closure plug/patch, collagen
- GMDN Term Description: An implantable, bioabsorbable device designed for haemostasis/closure of a puncture site, through pressure/compression, on a patient having undergone femoral artery catheterization; it is intended as an alternative to manual compression or surgical techniques to reduce the time to haemostasis. It consists of an animal-derived collagen plug that is implanted using an included delivery device (e.g., guidewire, hand-held delivery unit) onto the extravascular surface of the femoral artery access site (top of arteriotomy site), and may be held in place with an included synthetic, bioabsorbable patch/anchor/suture to achieve haemostasis.
Terumo
Terumo is a global leader in medical technology, offering innovative solutions across interventional systems, cardiovascular care, and blood management. With a focus on advancing patient outcomes, Terumo provides high-quality devices designed to support minimally invasive procedures and improve healthcare delivery.
Their extensive portfolio includes Glidewire® guidewires for vascular access, the Angio-Seal® closure device for safe vascular sealing, and the Misago® peripheral stent system for treating peripheral artery disease. Committed to precision, reliability, and innovation, Terumo empowers healthcare professionals to deliver exceptional care in interventional and surgical specialties worldwide.









